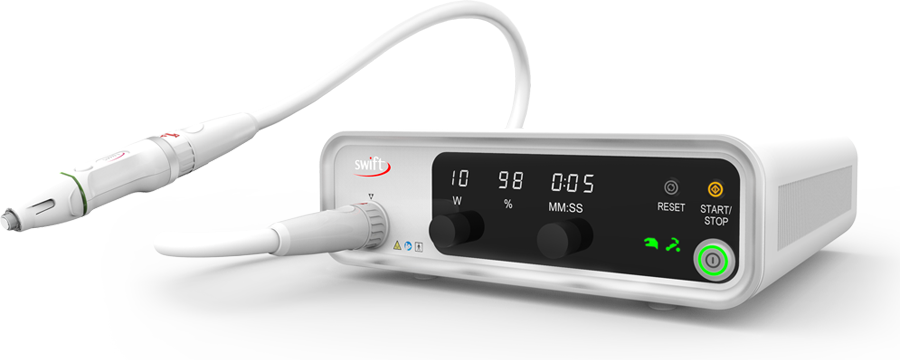
Swift has been developed in the UK and in October 2018 received FDA clearance in the United States of America. Swift delivers low-level microwave energy along a specialist cable, through a handpiece, and onto a verruca or wart. As its name suggests the treatment itself is swift, often no longer than two seconds per application.
Swift microwave therapy delivers a consistent dose of energy over a consistent period of time to a pre-determined depth; the treatment, which is intended to coagulate soft tissue during a non-invasive procedure, is clean with very little need of post-operative dressings; the procedure can be carried out routinely in clinic for patient convenience; and the patient can be confident in the treatment based on independent research and results.
More information can be found by clicking here.




